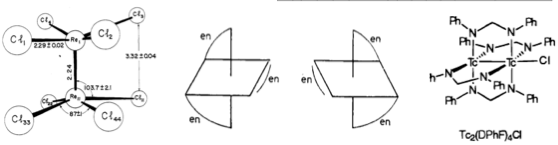
I like trends, I like rules, I like to explain things using them. The moment I saw this table of rate constants, I fell in love with it. The study was done by Manfred Eigen and his colleagues and I think it is brilliant. Here is the table and the paper :
The main trend is obvious. In the same group, the rate of exchange increases with the size of the ion.
There are also other very important trends and exceptions that he lists in the paper in case you are interested. The great thing is that they can be explained by certain rules that you learn in undergraduate inorganic chemistry courses. I love it!
Monday, December 30, 2013
Berzelius and How Is the Laboratory Glassware made ?
Jöns Jacob Berzelius, who is known for the discoveries of silicon, selenium, thorium and cerium, is one of the greatest names in chemistry. But, in fact we owe him a lot more than that. He believed that the alchemical symbols for the elements made chemistry hard to learn and perform.
This idea was also adopted by Dalton. Even though Dalton tried to change the symbols of the elements, they were again hard to use in chemistry. Chemistry needed something simpler. Since chemists (and of course alchemists) used different symbols all over the world, it was almost impossible to read each other experiments. This was also one of the reasons why chemistry was not able to be conducted systematically. Understanding this fact and knowing that he was the one to change it, Berzelius decided to develop a nomenclature for the elements. He wrote "The chemical signs ought to be letters for the greater facility of writing, and not to disfigure a printed book. I shall therefore take for the chemical sign the initial letter of the Latin name of each chemical element, If the first two letters be common to two metals I shall use both the initial letter and the first letter they have not in common." By the help of this giant step, chemistry suddenly became more available to public and scientists. The reactions which were written in some kind of esoteric language was now clear and universal. This paved the way for the new chemists.
Berzelius was a great experimenter. He developed fine techniques to measure the atomic weights of the elements and they are remarkably close to the values measured with the state of the art instruments.
What made him so good at experimentation was his great skill at glassblowing and patience. He designed and made his glassware himself. This helped him to do more precise and accurate experiments. Even Goethe wanted to learn glassblowing from him.
I have always thought that all the glassware we use in the labs are mass production. We went on a field trip to a glassblowing shop and I was so surprised to see that majority of the glassware in the labs are made by glassblowers. The glassblower said that they make almost everything for the labs except beakers, test tubes etc. Obviously, they are now made by big companies. The type of glass used for the labs is called Pyrex-glass. Here is some more information about glassblowing and a comparison of different types of glasses :
A glassblower is definitely an artist. Although the one we visited makes only scientific glassware, he has all the skill that an artist needs. He also made a very small cute cup with a "smiley" on it. After the glassblowing finished, he showed us how he checks for leaks on the glass. They use Tesla Coil for it. You can watch how he made a "condenser" for us and then checked it by his Tesla Coil below :
Next time when you use your condenser or the gas line please remember that it was not made in a huge factory. Somebody really blew into glass and did it.
Sources :
1. Crucibles : Jaffe, Bernard. The Story of Chemistry Bernard. New York : Dover Publications Inc., 1976. Print.
2. Strathern, Paul. Mendeleyev's Dream. New York : Berkley Books, 2002. Print.
Related posts about chemistry books and chemists:
Dmitri Mendeleev (February 8, 1834-February 2, 1907)
Dmitri Mendeleev not only created the first periodic table, but also predicted elements that didn't exist at that time. At first, just like every great mind in history the scientific society didn't want to accept his great discovery. But, he was so good at calculations that soon after, no one dared to doubt his Periodic Law. For instance, he questioned Thorium's atomic weight and claimed that it was calculated wrong. Because, he predicted that it was different than what it was known and he was right !
When no element fitted to his table, he left an empty space. He calculated those undiscovered elements' atomic weights and some other properties. Two of these are Gallium and Germanium. When gallium was discovered, Lecoq de Boisbaudran (the discoverer) reported a different atomic weight for it. Mendeleev sent a letter to him and asked him to repeat his experiments and check his calculations. In the end, he was right ! Germanium's discovery came after many years and again confirmed Mendeleev's law.
He was a man of science. He worked with Bunsen in Heidelberg Laboratories and left the work after an argument with Bunsen. He attended lectures of Kirchhoff. His doctoral thesis was on solubility of alcohol in water. He spent many years in teaching chemistry, he helped to develop the oil industry in Azerbaijan wrote an inorganic chemistry book.
He deeply believed in science. He didn't like literature "We could live at the present day without a Plato, but a double number of Newtons is required to discover the secrets of nature, and to bring life into harmony with the laws of nature."
I think what made him different was his bravery and determined mind. Even if he had doubts, he defended his great discovery. He believed it to be true and science has proven he is right.
"...although I had my doubts about some obscure points, yet I have never doubted the universality of this law, because it could not possibly be the result of chance."
Happy Birthday Mendeleev !
Here is a good reading on Mendeleev and his great discovery by Eric Scerri ( @ericscerri ): http://blog.oup.com/2012/08/how-exactly-did-mendeleev-discover-his-periodic-table-of-1869/
Related posts about chemistry books and chemists:
The Double Helix by James D.Watson
I just want to write how I feel about this book and I don't want to make it look like a book review. It's the short history of DNA told by its discoverer.
First of all, I was surprised to learn that just like Max Dellbruck and Maurice Wilkins, Francis Crick was also a physicist and became interested in biology later in his career. You might think that changing fields is craziness and it only works for exceptional figures like those mentioned. Well, I used to think the same. But, after reading how much time and effort Francis Crick spent on thinking about DNA, I changed my mind. He wasn't a genius. He wasn't one of the greatest minds of his age and according to Watson, he wasn't even favored by his fellow scientists including his director Sir Lawrence Bragg. There were several occasions that they seriously considered sending him away.
Secondly, I was totally surprised that the four people (Maurice Wilkins, Rosalind Franklin, Francis Crick and James Watson) who were involved in the discovery of the helix, had many arguments. Surprisingly, Rosalind Franklin was not much of a fan of sharing her experiment results and data with others.
Maurice Wilkins was not so different. When Linus Pauling wrote him a letter and asked for a copy of X-Ray photos of the structures, he didn't send him any.
Linus Pauling wrote in his The Nature of the Chemical Bond; "It has been recognized that hydrogen bonds restrain protein molecules to their native configurations, and I believe that as the methods of structural chemistry are further applied to physiological problems it will be found that the significance of the hydrogen bond for physiology is greater than that of any other single structural feature. " He was absolutely right. During the time Crick and Watson spent on thinking of a possible structure for the DNA molecule, they were well aware of the fact that hydrogen bonding would help to stabilize the molecule. So, they tried several pairings of the base pairs and being good at physics and maths Francis Crick calculated the distances, bond lengths etc. They knew that Linus Pauling was a great chemist and being the discoverer of the alpha helix, he had the upper hand. Actually, Watson bought himself a copy of The Nature of the Chemical Bond to learn more about inorganic ions, bonding etc.
Francis Crick's tricks to deceive the "fellowship electors" is another story. He didn't really work where he was supposed to before he came to UK. I will not go into detail but I will just quote this one "It made me feel slightly dishonest as I set off for the sun."
He was also not interested in biochemistry. HE was actually sent abroad by his "boss" to learn biochemistry but he didn't really like it. This was my biggest surprise and may be he had to tell lies about his workplace and research interests. I think there is enough evidence in the book to answer this question and I believe he really had to do so.
There are also some evidences how women in science were perceived. So, I can empathize with R.Franklin to some extent. I want to quote Watson here again ; "The thought could not be avoided that the best home for a feminist was in another person's lab."
I finished the book in a few hours and I seriously wished that it had 200 more pages. It was fascinating and I really loved it. I think I've learned so many important facts from this book that I will never forget in my future life and career. After all, I think it is the most famous molecule in the world.
The Periodic Table: A Very Short Introduction by Eric Scerri
I have read several books on the Periodic Table and Chemistry before such as: The Disappearing Spoon, Napoleon's Buttons, Mendeleyev's Dream and Nature's Building Blocks. The Disappearing Spoon where the story of each element is told and Napoleon's Buttons that gives brief information about 17 "famous" molecules are very popular. Mendeleyev's Dream is more like a history of chemistry from the early ages to the modern times and the stories of famous chemists and the discovery of elements are very well placed in the book. Nature's Building Blocks is a kind of "element" encyclopedia where you can find technical and historical information about elements. So, prior to reading Eric Scerri's book, I was expecting a book similar to the ones I'd read before. Well, it is really different if you think like I did.
- http://www.barnesandnoble.com/w/periodic-table-eric-scerri/1103079928?ean=9780199582495
The book starts with ancient Greeks and their conception of "elements." Then the author brings us to the Middle Ages and modern times. First five chapters of the book is mainly about the discoveries of the elements and the development of the Periodic Table where he gives information about the periodic law and illustrates different forms of the periodic table. Although I've read a few books about the Periodic Table and the elements as I mentioned above, I still learned quite a few new chemists and their efforts to organize the elements known during their lifetimes. Some of them in fact were very successful. Lothar Meyer for example was very close to be the "father" of the periodic table.
Fifth chapter is about Dmitri Mendeleev and his genius. I will not go into detail what he did. But, I think one of the most distinguishing parts of the book is this chapter where I've learned that Mendeleev also made failed predictions. I've never read his failures before.
Starting with the chapter six, the author goes into more physical and chemical theories and facts about the periodic law and the table. He tells how the physicists actually helped chemists to organize the periodic table by the theories and experimental evidences. I think these chapters are a nice review of general chemistry course that we are all supposed to take.
Towards the end of the book, you can find really brief but satisfying information on the electronic structure of an atom, orbitals, quantum mechanics, and how new elements are synthesized. Actually, I loved one of the figures in the book. It is a figure where the experimental ionization energies are compared with the theoretical calculations. I've never seen it before and I think it is very useful to see how close they are.
The final chapter is about different forms of periodic tables where you can find really interesting arrangements of the elements in "short, " "medium long" and "long" forms of the periodic tables. Honestly, I have always wondered which one of these periodic tables is more "correct."
- http://www.chemistryland.com/CHM151W/02-Atoms/Chaos/PeriodicInnerTrans.jpg
I will not give the answer and if you are curious you can read the book and learn.
I think it's a really well organized and useful book for chemists and students studying chemistry. I strongly suggest that you should read it. You might think that the technical information might discourage people from other areas or a "normal" popular science book reader from reading the book. But, considering that a lot of quantum mechanics books are "popular" and "best sellers," I think any reader can read it and understand most of it. The fact that it has a small size is also another factor that it is a easy-to-read (or handle) book. I also guarantee you there are many figures in the book that you have never seen before.
Book: Mendeleyev's Dream
This book is very similar to this one that I have written about before. I think the biggest difference is that Mendeleyev's Dream does a better job in explaining how periodic table formed and the author writes about fewer chemists throughout the history.
The quote in the beginning of the book is fascinating:
The quote in the beginning of the book is fascinating:
"The chemists are a strange class of mortals, impelled by an almost insane impulse to seek their pleasures amid smoke and vapour, soot and flame, poisons and poverty; yet among all these evils I seem to live so sweetly that may I die if I were to change places with the Persian king."
— Johann Joachim Becher, Physica subterranea (1667)
Book: What is Life? How Chemistry Becomes Biology
I bought this book thinking that it was about chemistry and biology. But, I was wrong. This book is more of a philosophical approach to science (chemistry&biology). So, if you are looking for a simple answer to the question in the title, you will probably be disappointed. For the first 160 pages, the author lays down the basics of science and research and only in the final chapter he tries to answer the main question.
If you don't have a biology or chemistry background, it might be a heavy book. But, for the people who know a little bit about them and who are interested in fundamental questions, it is a good book.
If you don't have a biology or chemistry background, it might be a heavy book. But, for the people who know a little bit about them and who are interested in fundamental questions, it is a good book.
Book: Metals in Biochemistry
I love bioinorganic chemistry and the role of metal ions in biology. So, whenever I get the chance, I read a textbook, publication or even popular science articles about the metals in biology and medicine. As soon as I saw this book on abebooks.com , I bought it. It's a very short (75 pages) book about the a few metals in biology such s iron, cobalt, molybdenum, zinc etc. The one I have was published in 1980, so lots of things have changed since the publication of the book. But, as the foreword suggests, this book is not a textbook. It is just an introduction about several metal ions that are essential for life as we know it.
Book: Nature's Building Blocks
This books is more like an encyclopedia of elements. Because the elements are ordered in alphabetical order in this book, it is hard to see the similarities between elements. Each chapter(element) gives brief information about the element's history, discovery and use in industry, medicine or food etc.
There is a table in the end of the book that shows the discovery(or synthesis) of the elements in chronological order. I think it is really helpful.
Book: From Caveman to Chemist
I have recently read this book. Like many other "popular" chemistry books, it starts with the ancients and their conception of elements and takes us to 1900's.
If you want to have some general information about the roots of chemistry, ancient, Greek, Roman, Islamic concepts of chemistry and how it became a major discipline after the medieval age, this is a nice book.
If you want to have some general information about the roots of chemistry, ancient, Greek, Roman, Islamic concepts of chemistry and how it became a major discipline after the medieval age, this is a nice book.
Book: Crucibles:The Story of Chemistry
This is an excellent book that shows the progress of chemistry from 15th century to 20th century. Each chapter is about one single chemist and decorated with the details of their lives and research and I think this is what makes the book special.
Book: The Disappearing Spoon
I think it is one of the most popular books about chemistry and the elements. I read it almost 2 years ago and I really enjoyed it. The author tells at least one interesting story about each element on the periodic table. While doing this, he also introduces atomic theories, some chemistry and physics and even some biology.
The only thing I don't like about this book is that the chapters don't follow the groups on the periodic table or increasing atomic number.
The only thing I don't like about this book is that the chapters don't follow the groups on the periodic table or increasing atomic number.
Summer research
I was lucky enough to be accepted for a 10 week paid summer research in my school lab. I wasn't too excited, because I had spent 2 semesters doing research. The problem with the research during the school semesters was that I didn’t have enough time between reactions. Sometimes, I had to leave the lab right after I had isolated my product and came back 2 days later without knowing of my compound is still the same! So, I was very confident before starting my research this summer. I was sure that in 10 weeks I could do great things in the lab. Apparently, I was wrong.
I am working in a synthetic (bio)inorganic chemistry group and I have my own project. So, I make my own ligands, bind them to metals and so on(we all do the same by the way). First problem came by the first ligand. Since it is a known ligand, I can give the name: TACN (1,4,7-Triazacyclononane). I don’t want to go into detail, but no matter how hard I tried, I wasn’t able to make it. I’ve spent so much time reading papers describing the synthesis. I followed the same methods, modifies a little bit, did it under Argon etc. But, it didn’t work. By the way, I have found several papers with flawed data etc. If you look at the structure and synthesis, it doesn’t really look hard and I am sure for an organic chemist it is very easy. But, I’ve now learned that making macrocycles is extremely hard in some cases. So, we had to spend some money and buy the commercial one. I lost 5 weeks with this synthesis (or I’ve gained 5 weeks of experience).
After making my other ligand (which I can not name here), finally I started to do reactions with my new friend Iridium. Since I have been trying to make a new metal complex, I didn’t have procedure to follow. So, I brought in my own knowledge of organic and inorganic chemistry to work. I read papers on similar metal complexes of Rhodium. Because, one would expect that they should “behave” similar(they really don't). Of course, my mentor helped me a lot. But, most of the time he pushed me to decide on my own. So, for the first time I was in a position that I was afraid to make mistakes. Because, they were my OWN decisions and I felt responsible for them. I dealt with extremely insoluble intermediates and products. I had to find ways to characterize them and compare different spectra. Basically, the last 5 weeks went like this. Another big problem was the yields I was getting (usually 30-40%). So, I tried to maximize yields by changing the reaction conditions etc. Finally, my time was over and I couldn’t even reach half of my goals that I wrote in my proposal. I have gained a lot of experience and I will make us of them in the future. I have obtained more than 100 NMR spectra. So, I am very confident in NMR interpretation now. I have also helped my friends in other groups in finding their resonances. I kind of became the NMR guy.
In summary, now I know why a Ph.D. takes 4+ years. I now know a reaction that’s suppsed to go 3 hours can keep you busy for 3 days (isolating, characterizing etc.). I now know how hard chemistry is. I now know that NMR, IR, UV/Vis don't tell you anything unless you know how to read them and you are well aware of what you are looking for. I now know how frustrating it can be. I now know silver makes stains on your skin! And now once again I really know I want to be a chemist. Because, it is so exciting!
Plutonium and the movie: Pu-239
Plutonium is one of the actinides and it was discovered by Glenn T.Seaborg and his team in 1941. Neptunium was named after Neptune (the first planet beyond Uranus) and therefore plutonium was named after Pluto (the second (ex)planet beyond Uranus).
At first, the amount of the plutonium produced was too little to record the mass. The first recorded mass of a plutonium compound was 2.77 micrograms. Shortly after its discovery, it was realized that one isotope of plutonium can undergo a nuclear chain reaction and it could be used in making an atomic bomb. This isotope was Plutonium 239. So, Plutonium Project started under the pseudonym of Metallurgical Project. This lead to a process of producing huge amounts of plutonium and finally, in 1945 a plutonium containing nuclear weapon, Fat Man was dropped on Nagasaki killing 70000 people.
More plutonium : The Plutonium Story : The Journals of Professor Glenn T.Seaborg
Today, plutonium is mostly used in nuclear power plants.
A few months ago, I watched a movie called Pu-239.
----Spoiler alert!----
Pu-239 is a striking movie in several aspects. First of all, it is a love story. A man (Timofey) has been exposed to plutonium and knows he will die soon. The administrative staff of the plant wants to cover the leak. So, they try to buy him off. Having no other option than stealing some plutonium from his work to help his family, he leaves for Moscow to find a buyer.
Secondly, it is a story of corruption. The movie takes place in Russia at a time when mobs rule the cities, businesses. It's a period of poverty. The market scene and later a robbery clearly shows the socio-economic status of the city or country.
Finally, it is a wonderful movie because it has chemistry in it! I also love the script, because right after the scientific entries, there are very deep sentences. There is also a reference to Radium Girls.
"Light is a particle and a wave...Women, children and light can be two things at once; a particle, a wave. They ricochet off the hard surfaces and illuminate the corners. Without them it would be far darker."
"An element loses a particle and becomes unstable. A chain reaction is set in motion. Pulsing waves of desperation in every direction.Perhaps the lost part is clarity or hope."
"Are you aware of the radiological effects on living organisms? Protons cut through your DNA rewriting your genetic code."
"Uranium, Neptunium, Plutonium. They came from space; found their way here by comet and meteorite. No child ever wished this from a star. Hiroshima, Nagasaki, Chernobyl. Problems with half-lives forty-thousand years long. "
" In the end, everything decays to lead, number eighty-two on the periodic table. All of the brilliant things born in the center of stars will have turned cold and gray. Everything is moving in that direction. Toward lead. Impossible to stop."
I strongly suggest that you should watch this movie. I can not think of one single chemist that won't like it. It is a shame that this movie is not widely known and the imdb rating of 6.8 is another reason for me to hate populism.
Surface Tension
Surface tension can be described as "the tendency of liquids to minimize their surface area." The molecules closer to the surface of a liquid has higher potential energy. This means that the molecules deeper than the ones close to the surface, has to gain energy in order to move upwards to reach the surface. Since the molecules want to have lower energies to be more stable, they want to stay closer to each other by making the surface area smaller. The decrease in intermolecular forces causes surface tension to decrease too.
Everybody must have observed that water droplets are spherical. The larger the droplets become, they are more effected by the gravity and lose their shapes.
Check it out what happens at zero gravity :
Shaking the Coke under higher pressures
We all know what happens to a can of coke when we shake it and open the can. What do you think happens under 2.5 atm ? Check it out :
You might also want to read about Henry's Law .
Subscribe to:
Posts (Atom)