A friend was making some starting materials and [they] allowed me to take some photos of these beautiful compounds. Bright colors are definitely among the top reasons to love inorganic chemistry.
Sunday, March 22, 2015
Saturday, March 21, 2015
Technetium Chemistry in the Fuel Cycle: Combining Basic and Applied Studies #chempaperaday 204
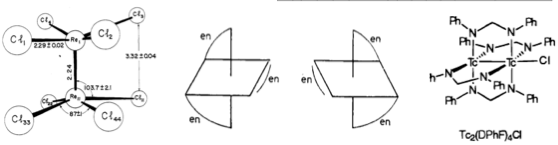
Technetium is the most mysterious element. In the middle of the d-block and yet radioactive. You can't just dig and find technetium (therefore the name). Despite all these difficulties, the isotope 99mTc is the "workhorse of diagnostic nuclear medicine." It is the byproduct of fission reactions of uranium isotopes in the reactors. Because, it requires special handling and other requirements, not every lab is lucky to study technetium chemistry. So, not much is known about it yet. All I know is that it makes nice bimetallic complexes with multiple Tc-Tc bonds. So, I was not much surprised to learn that "[on binary compounds]...only three species, TcF6, TcF5, and TcCl4, identified prior to 2008."
A few more details from the paper:
A few more details from the paper:
Because of its high fission production rate, the amount of technetium on Earth may ultimately exceed that of rhenium, its heavier congener.
In a typical light water nuclear reactor, 99Tc production is around 2 g/day for every 100 MW of thermal energy.
Technetium has a range of oxidation states, from 7+ to 1–, and its chemistry has considerable similarities to rhenium; e.g., it has extensive pentavalent chemistry.
The overall trends clearly demonstrate increasing technetium–technetium interactions with decreasing oxidation state.
A Half-Century of Nonclassical Organometallic Chemistry: A Personal Perspective #chempaperaday 203
The 100th #chempaperaday was "A millennial overview of transition metal chemistry" by F.A. Cotton. Interestingly enough, my 203rd paper is another great article by him.
In this article, he highlights a few selected areas of inorganic chemistry and obviously his contributions to these areas. In fact, he paved the way for several of these. When chemists just put their hands on an NMR instrument, he started to do VT experiments to study the fluctional behavior of several organometallic compounds. Incredible work. There is also a few pages on the iron dimer that I just posted, the discovery of agostic interactions, his proposal to use hapticity and so on.
"Prior to the report on ferrocene, no one worked in the field of transition metal organometallic chemistry because no one believed that there was any such field to work in."
Does cyclopentadienyl iron dicarbonyl dimer have a metal–metal bond? Who’s asking? #chempaperaday 202
A really nice article on why we should draw a bond between iron atoms in this complex. I presented this paper later to a group of people. I defended Jay Labinger's view and my own literature search and the evidence (homolytic cleaveage to give 17 electron radical species etc.) I saw made me think that we should draw a bond. If you like controversies and discussions on transition metal complexes like me, you will enjoy it.
I should note that the article has quotes from Galileo, poems from Dante and Pushkin. It is not very often you see these types of things in articles which made me like the article even more.
http://www.sciencedirect.com/science/article/pii/S0020169314002278
A few quotes:
I should note that the article has quotes from Galileo, poems from Dante and Pushkin. It is not very often you see these types of things in articles which made me like the article even more.
http://www.sciencedirect.com/science/article/pii/S0020169314002278
A few quotes:
..given Al Cotton’s intimate involvement with the history of both M–M bonds in general and Fp2 in particular – not to mention his fondness for engaging in polemics.
The latter argues against E. O. Fischer’s description: probably this constitutes Cotton’s first polemic in print!)
The metathesis reactions: from a historical perspective to recent developments #chempaperaday 201
A nice historical perspective of olefin metathesis and metathesis catalysts. The article was published in 2005. So, don't expect to see exciting new stuff like enantioselective ones.
I remember writing on metathesis before. So, let's add some new things that I learned.
I remember writing on metathesis before. So, let's add some new things that I learned.
"..the first observation of the metathesis of propene at high temperature was reported in 1931.
Schrock, then at Du Pont, tried to synthesize [Ta(CH2CMe3)5], which would not contain b-hydrogens and thus, according to this principle, should have been stable. Good luck smiles only on good scientists, and the expected compound did not form...led to the isolation of the first stable metal-alkylidene complex, [Ta(CH2CMe3)3(=CHCMe3)]."
"Exploration of Earth-Abundant Transition Metals (Fe, Co, and Ni) as Catalysts in Unreactive Chemical Bond Activations" #chempaperaday 200
This is a short article on Fe, Co and Ni catalysts towards C-O, C-C and C-H activation. My purpose to read this article was to see "what is out there" and see if I can learn a few new things. I want to point out a few important (at least for me) details in this article on the characteristic behaviors of these metals. As an inorganic chemist, I enjoyed these parts the most.
Fe : Let's repeat that it is the most abundant transition metal on earth.
Co : Like iron, it is cheap and abundant. But, in these type of bond activation reactions, Co catalysis "lagged greatly."
Ni : $1/g ! Unbelievable! The congeners of Ni; Pd($183/g), Pt($483/g)! Oxidative addition of Ni(0) or Ni(I) usually goes through SET processes. And as we can see from the beautiful periodic table, Ni is more nucleophilic as expected. The authors also claim that Ni is "the best metal to induce the C-O cleaveage of various O-based nucleophiles."
There are lots of examples of bond activations and catalytic cycles in the paper. I noticed an error in one of the figures:
Also, one of the authors was born in 1991 and he is a ""third year graduate student."
Fe : Let's repeat that it is the most abundant transition metal on earth.
Co : Like iron, it is cheap and abundant. But, in these type of bond activation reactions, Co catalysis "lagged greatly."
Ni : $1/g ! Unbelievable! The congeners of Ni; Pd($183/g), Pt($483/g)! Oxidative addition of Ni(0) or Ni(I) usually goes through SET processes. And as we can see from the beautiful periodic table, Ni is more nucleophilic as expected. The authors also claim that Ni is "the best metal to induce the C-O cleaveage of various O-based nucleophiles."
There are lots of examples of bond activations and catalytic cycles in the paper. I noticed an error in one of the figures:
Also, one of the authors was born in 1991 and he is a ""third year graduate student."
"A Nickel(II)–Sulfur-Based Radical-Ligand Complex as a Functional Model of Hydrogenase" and the "controversy" #chempaperaday 197-199
The first paper came out in 2010 and reported synthesis and characterization of a Ni(II)-dithiolene complex. The authors formulated the compound as NiII(L2−)(L−.)][PPh4] and claim that complex "shows strikingly similar EPR and reduction potential values to those observed with native Ni-containing hydrogenases." I should mention that CV of the compound was reported in MeCN. You can read the paper and ask questions, but the most interesting thing they did not provide a proton NMR spectrum for this paramagnetic compound. But, they assigned a signal at 4.1 ppm to a S-H. Interesting.
Anyway, shortly after a "comment" was published and proposed a new oxidation state assignment for the Ni atom and the ligand. A "Response" followed and honestly I don't think they added any new evidence or supporting argument for their own assignment.
I still think, they are worth reading.
Sunday, March 8, 2015
Book: The Violinist's Thumb
I got this book as a gift. As I read The Disappearing Spoon by the same author before, I was expecting it would be as entertaining and interesting as it was. I think this is a good book to read, but if you already know some about biology, genetics, genes and some evolution; I don't think you will learn much. I read it quickly and I liked it (3 stars). There are several interesting stories, but in my case, I knew most of them. Anyway, I still think it is worth reading since there are a lot of names and details. Also, you will have a lot to learn if you do not know much about science.
Friday, March 6, 2015
A Salute to My Colleague Yves Chauvin, 1930–2015 #chempaperaday 196
Olefin metathesis is one of the most important and useful chemical transformations. The earliest versions of this reaction involved using not well defined catalysts and the reaction mechanism was not understood. In 1971, Chauvin proposed a mechanism that is accepted as the actual mechanism of the reaction today.
 Chauvin died on January 27. I have read about him before. But, I think this is the best article.
Chauvin died on January 27. I have read about him before. But, I think this is the best article.
Here are a few quotes from him proving how good a scientist he was:

Here are a few quotes from him proving how good a scientist he was:
“If you want to find something new, look for something new!”
“Some people say that there is too much chemistry in our society. It is in fact the opposite: there is not enough chemistry, not enough understanding, not enough control of chemical reactions. Catalysis is the key for a better chemistry.”
Subscribe to:
Posts (Atom)