When I read a book and if like it, I try to buy it. I really
love this book, but even the used ones start from $88 on Amazon. So, it looks like I will not be able to own this book for some time. But, I tried to write down as much as possible for my notes until I buy one.
So, the book is about four different methods as you can understand from the title. As the author says in the preface, the book "is not a spectroscopic textbook, nor is it written for those with a need for detailed theory." There is really very little about the theory of the techniques and they were kept as simple as possible. I was able to understand almost everything without any further reading or help.
To be honest, I don't remember seeing any NQR spectra in publications and that's the only chapter I didn't pay much attention.
What I like the most about the book is the chapter problems which are mostly from journal articles. So, you are given a spectrum and asked to interpret it. Or you are given the complex and asked to make an educated guess on how the spectrum should look like. Or calculate isomer shifts, g values etc.
I wrote a post about one of these simple problems
here. After studying the chapter on NMR, I dived into some papers and tried to apply what I learned. I am happy that I was able understand them better.
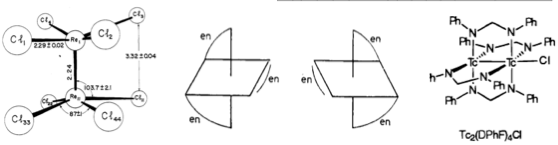
I have also just finished two Mossbauer spectra posts and right now I am trying to read some literature so that I can write a longer and more detailed discussion for my posts. I also discovered a fascinating story on the determination of a molecular geometry for a transition metal complex. It is really amazing and I loved it. I will write a summary of the story and link all the published data and discussions in a post hopefully this weekend.
In summary, I think this book is a must read/study book for a student like me. If I were teaching inorganic chemistry and spectroscopy, I would also ask similar problems in my exams.