Monday, February 24, 2014
Friday, February 21, 2014
#realtimechem (9 AM) precipitation
Another reason to love chemistry. Isn't it magic?
Life...
In case anyone is reading this blog, I just got caught up with life again. There are decisions to make and a road ahead. I need lots of thinking and planning.
Tuesday, February 18, 2014
Did R.S.Mulliken sign and write in this book?
As I said in this post, I ordered a copy of "Electrons and Chemical Bonding" by Harry B. Gray and I have just received it. When I opened the first page, I saw this:
First thing I thought was if the signature belongs to R.S. Mulliken or not. I tried to find his signature on google images and these are the most relevant images:
First of all, I want to believe that the signature belongs to him.
I love buying used books and one of the reasons is that I like reading the notes of the previous owners. There is a good chance that the signature is fake or the signature belongs to Mulliken but the notes do not. Still, it is an interesting day for me. I hope chemists (and physicists) and historians of chemistry(and physics) help me to find out the truth.
But the story is not finished yet. The book is full of notes, questions, comments, arrows etc. It is obvious whoever took notes on this book is someone who knows chemistry and physics enough to notice details and ask questions. But, I can not read most of the notes since they are written in a kind of handwriting. I am sure any native English speaker can read them easily though. Here are some of them:
Monday, February 17, 2014
#chempaperaday Day 31/365: "A platinum complex that binds non-covalently to DNA and induces cell death via a different mechanism than cisplatin"
This article is an open access article about a Pt(II) terpyridine complex and its interaction and binding mode with DNA.
The studies showed that the complex can enter the cell nucleus and interact with DNA in a non-covalent manner. They also found that the complex has a selective cytotoxicity towards certain cancer lines.
I have been trying to follow metal complex-DNA interaction papers. But, looks like I missed this one. It's a great study. But, I would like to know why there is no x-ray data.
My reading list (89 books in my Amazon shopping cart)
So, I decided to post the list of the books (including textbooks)in my amazon shopping cart. I think it is a good reading list that other people can find something interesting too. Note that the order is not an order of importance. I tried to keep them in order of chemistry, biology, popular science books etc., but then I got tired. Maybe I will organize them later.
1. The Mechanisms of Reactions at Transition Metal Sites
2. Inorganic Spectroscopic Methods
3. NMR Spectroscopy in Inorganic Chemistry
4. Introduction to Quantum Theory and Atomic Structure
5. Biocoordination Chemistry
6. The Ten Most Beautiful Experiments
7. d-Block Chemistry
8. The Kaiser Wilhelm Society under National Socialism
9. Neither Physics nor Chemistry: A History of Quantum Chemistry
10. Advanced Chemistry (Advanced Science)
11. The Improbability Principle: Why Coincidences, Miracles, and Rare Events Happen Every Day
12. Inorganic Syntheses (Volume 35)
13. The Biology of Cancer
14. Basic Physical Chemistry: The Route to Understanding
15. Metals in Cells
16. The Billion Dollar Molecule: One Company's Quest for the Perfect Drug
17. Physical Chemistry for the Chemical and Biological Science
18. Bringing Chemistry to Life: From Matter to Man
19. The Chemistry of Evolution
20. Oxygen: A Four Billion Year History
21. From X-rays to DNA: How Engineering Drives Biology
22. The Story of Earth: The First 4.5 Billion Years, from Stardust to Living Planet
23. Your Inner Fish: A Journey into the 3.5-Billion-Year History of the Human Body
24. Only a Theory: Evolution and the Battle for America's Soul
25. Evolution, Second Edition
26. Madame Curie: A Biography
27. The Monkey's Voyage: How Improbable Journeys Shaped the History of Life
28. The Theory of Intermolecular Forces
29. Cells to Civilizations: The Principles of Change That Shape Life
30. Introduction to Coordination Chemistry
31. Metal Complex - DNA Interactions
32. Metallomics and the Cell
33. The Secret of Scent: Adventures in Perfume and the Science of Smell
34. The Emperor of All Maladies: A Biography of Cancer
35. Gertrude Elion: Nobel Prize Winner in Physiology and Medicine
36. Handbook on Metalloprotein
37. Interrelations between Essential Metal Ions and Human Disease
38. Metals and Life
39. The Third Man of the Double Helix: The Autobiography of Maurice Wilkins
40. The Drug Book: From Arsenic to Xanax, 250 Milestones in the History of Drugs
41. Coordination Chemistry in Protein Cages: Principles, Design, and Applications
42. Mendeleev on the Periodic Law: Selected Writings, 1869 - 1905
43. Periodic correlations (The Physical Inorganic Chemistry Series)
44. Metals in Medicine
45. The Logic of Life
46. Chemistry - The Impure Science
47. Medicinal Inorganic Chemistry
48. The Annotated and Illustrated Double Helix
49. What Mad Pursuit: A Personal View of Scientific Discovery
50. The Plutonium Story: The Journals of Professor Glenn T. Seaborg 1939-1946
51. The Immortal Life of Henrietta Lacks
52. Letters to a Young Chemist
53. The Dark Side of the Enlightenment: Wizards, Alchemists, and Spiritual Seekers in the Age of Reason
54. The Secrets of Alchemy (Synthesis)
55. Distilling Knowledge: Alchemy, Chemistry, and the Scientific Revolution
56. Life's Ratchet: How Molecular Machines Extract Order from Chaos
57. SPECTROSCOPIC METHODS IN ORGANIC CHEMISTRY
58. Euler: The Master of Us All
59. Why Chemical Reactions Happen
60. Metallointercalators: Synthesis and Techniques to Probe Their Interactions with Biomolecules
61. Problems in Quantum Mechanics
62. Molecular Symmetry and Group Theory
63. Modern Physical Organic Chemistry
64. The Making of the Atomic Bomb: 25th Anniversary Edition
65. Stalin and the Soviet Science Wars
66. Why Does E=mc2? (And Why Should We Care?)
67. Albert Einstein's Vision: Remarkable Discoveries That Shaped Modern Science
68. Quantum: Einstein, Bohr, and the Great Debate about the Nature of Reality
69. Big Bang: The Origin of the Universe (P.S.)
70. Thirty Years that Shook Physics: The Story of Quantum Theory
71. QED: The Strange Theory of Light and Matter
72. Einstein: His Life and Universe
73. Benjamin Franklin: An American Life
74. Tesla: Man Out of Time
75. Radioactive: Marie & Pierre Curie: A Tale of Love and Fallout
76. Darwin: Portrait of a Genius
77. Marie Curie and Her Daughters: The Private Lives of Science's First Family
78. American Prometheus: The Triumph and Tragedy of J. Robert Oppenheimer
79. Uncle Tungsten: Memories of a Chemical Boyhood
80. The Clockwork Universe: Isaac Newton, the Royal Society, and the Birth of the Modern World
81. The Strangest Man: The Hidden Life of Paul Dirac, Mystic of the Atom
82. My Inventions: The Autobiography of Nikola Tesla
83. Erwin Schrodinger and the Quantum Revolution
84. The Los Alamos Primer: The First Lectures on How To Build an Atomic Bomb
85. The Science of Leonardo: Inside the Mind of the Great Genius of the Renaissance
86. Galileo: Watcher of the Skies
87. Gödel, Escher, Bach: An Eternal Golden Braid
88. The Red Queen: Sex and the Evolution of Human Nature
1. The Mechanisms of Reactions at Transition Metal Sites
2. Inorganic Spectroscopic Methods
3. NMR Spectroscopy in Inorganic Chemistry
4. Introduction to Quantum Theory and Atomic Structure
5. Biocoordination Chemistry
6. The Ten Most Beautiful Experiments
7. d-Block Chemistry
8. The Kaiser Wilhelm Society under National Socialism
9. Neither Physics nor Chemistry: A History of Quantum Chemistry
10. Advanced Chemistry (Advanced Science)
11. The Improbability Principle: Why Coincidences, Miracles, and Rare Events Happen Every Day
12. Inorganic Syntheses (Volume 35)
13. The Biology of Cancer
14. Basic Physical Chemistry: The Route to Understanding
15. Metals in Cells
16. The Billion Dollar Molecule: One Company's Quest for the Perfect Drug
17. Physical Chemistry for the Chemical and Biological Science
18. Bringing Chemistry to Life: From Matter to Man
19. The Chemistry of Evolution
20. Oxygen: A Four Billion Year History
21. From X-rays to DNA: How Engineering Drives Biology
22. The Story of Earth: The First 4.5 Billion Years, from Stardust to Living Planet
23. Your Inner Fish: A Journey into the 3.5-Billion-Year History of the Human Body
24. Only a Theory: Evolution and the Battle for America's Soul
25. Evolution, Second Edition
26. Madame Curie: A Biography
27. The Monkey's Voyage: How Improbable Journeys Shaped the History of Life
28. The Theory of Intermolecular Forces
29. Cells to Civilizations: The Principles of Change That Shape Life
30. Introduction to Coordination Chemistry
31. Metal Complex - DNA Interactions
32. Metallomics and the Cell
33. The Secret of Scent: Adventures in Perfume and the Science of Smell
34. The Emperor of All Maladies: A Biography of Cancer
35. Gertrude Elion: Nobel Prize Winner in Physiology and Medicine
36. Handbook on Metalloprotein
37. Interrelations between Essential Metal Ions and Human Disease
38. Metals and Life
39. The Third Man of the Double Helix: The Autobiography of Maurice Wilkins
40. The Drug Book: From Arsenic to Xanax, 250 Milestones in the History of Drugs
41. Coordination Chemistry in Protein Cages: Principles, Design, and Applications
42. Mendeleev on the Periodic Law: Selected Writings, 1869 - 1905
43. Periodic correlations (The Physical Inorganic Chemistry Series)
44. Metals in Medicine
45. The Logic of Life
46. Chemistry - The Impure Science
47. Medicinal Inorganic Chemistry
48. The Annotated and Illustrated Double Helix
49. What Mad Pursuit: A Personal View of Scientific Discovery
50. The Plutonium Story: The Journals of Professor Glenn T. Seaborg 1939-1946
51. The Immortal Life of Henrietta Lacks
52. Letters to a Young Chemist
53. The Dark Side of the Enlightenment: Wizards, Alchemists, and Spiritual Seekers in the Age of Reason
54. The Secrets of Alchemy (Synthesis)
55. Distilling Knowledge: Alchemy, Chemistry, and the Scientific Revolution
56. Life's Ratchet: How Molecular Machines Extract Order from Chaos
57. SPECTROSCOPIC METHODS IN ORGANIC CHEMISTRY
58. Euler: The Master of Us All
59. Why Chemical Reactions Happen
60. Metallointercalators: Synthesis and Techniques to Probe Their Interactions with Biomolecules
61. Problems in Quantum Mechanics
62. Molecular Symmetry and Group Theory
63. Modern Physical Organic Chemistry
64. The Making of the Atomic Bomb: 25th Anniversary Edition
65. Stalin and the Soviet Science Wars
66. Why Does E=mc2? (And Why Should We Care?)
67. Albert Einstein's Vision: Remarkable Discoveries That Shaped Modern Science
68. Quantum: Einstein, Bohr, and the Great Debate about the Nature of Reality
69. Big Bang: The Origin of the Universe (P.S.)
70. Thirty Years that Shook Physics: The Story of Quantum Theory
71. QED: The Strange Theory of Light and Matter
72. Einstein: His Life and Universe
73. Benjamin Franklin: An American Life
74. Tesla: Man Out of Time
75. Radioactive: Marie & Pierre Curie: A Tale of Love and Fallout
76. Darwin: Portrait of a Genius
77. Marie Curie and Her Daughters: The Private Lives of Science's First Family
78. American Prometheus: The Triumph and Tragedy of J. Robert Oppenheimer
79. Uncle Tungsten: Memories of a Chemical Boyhood
80. The Clockwork Universe: Isaac Newton, the Royal Society, and the Birth of the Modern World
81. The Strangest Man: The Hidden Life of Paul Dirac, Mystic of the Atom
82. My Inventions: The Autobiography of Nikola Tesla
83. Erwin Schrodinger and the Quantum Revolution
84. The Los Alamos Primer: The First Lectures on How To Build an Atomic Bomb
85. The Science of Leonardo: Inside the Mind of the Great Genius of the Renaissance
86. Galileo: Watcher of the Skies
87. Gödel, Escher, Bach: An Eternal Golden Braid
88. The Red Queen: Sex and the Evolution of Human Nature
Not every dream comes true
If you don't know it yet, I am a senior undergraduate chemistry student and graduating in less than 3 months. I started my studies knowing that an undergraduate level of knowledge in chemistry would never be enough for me. I want to know more chemistry and I will try to keep learning more no matter how much time it takes to satisfy myself.
Like everyone else, I had a dream too and it was to have my Ph.D. in chemistry at MIT. I will not give names here who I really wanted to work for though. Anyway, so I applied to MIT and I got rejected from my dream school. Does it hurt? Yes, it does. Do I know why I got rejected? Of course not. But, I can think of a few reasons for my rejection. After all, it is the most famous school in the world and thousands of people from tens of countries apply each year. So, I don't feel too bad. I know I lost my chance to people who are great. You can say that I still have a chance to do a post.doc. there, but it won't be the same.
Anyway, I had to write.
PS: If anyone is wondering, yes I have been accepted to a few schools. But, I will not announce my final decision until I accept an offer and make it official. But, I can say that I have been having one of the most challenging periods of my life. I guess the second most challenging.
Sunday, February 16, 2014
#chempaperaday Day 30/365: "Discovery of Novel Small-Molecule Inhibitors of BRD4 Using Structure-Based Virtual Screening"
I love inorganic chemistry. But, if I had a copy of me, I would make him go for medicinal chemistry. I think it is great to be involved in a research field that has an extremely important role in medicine. So, today I read this paper:
http://pubs.acs.org/doi/pdf/10.1021/jm4011302 (open access)
I hope you like it too.
Obviously, I do not know anything about medicinal chemistry. But, I hope I know enough biochemistry and organic chemistry to assist me in understanding the very simple parts of a publication.
With the help of this paper, I also learned (I guess) a few methods in medicinal chemistry. So, I think one method is to do a kind of literature/structure search and a second method is to change (add/remove) functional groups on a molecule. I could be totally wrong too. I hope I am not. Also, it is obvious that medicinal chemists try to design the drugs so that they will fit into the "pockets" or the targets on a protein etc. That sure makes sense. Then chemists come up with probably hundreds of candidates and computational chemists test them in silico before any experiments are done (this is my guess).
One thing I noticed that in "conclusion," it is written "bromdomain" while "bromodomain" is used in the rest of the article. I guess it is a typo.
Saturday, February 15, 2014
#chempaperaday Day 29/365: "Biosynthesis of the Urease Metallocenter"
I am dying to know how metalloproteins are "made" and folded in the cell. When do the metals take their places? How is the concentration of the metal ion is regulated? What kind of proteins or molecules helped them to enter the cell and how do they end up where they belong?
This article is a review about urease; a nickel containing metalloprotein.
http://www.jbc.org/content/288/19/13178
http://www.rcsb.org/pdb/explore/explore.do?structureId=1FWJ
This article is a review about urease; a nickel containing metalloprotein.
http://www.jbc.org/content/288/19/13178
http://www.rcsb.org/pdb/explore/explore.do?structureId=1FWJ
Friday, February 14, 2014
#chempaperaday Day 28/365: "Metal−Organic Proximity in a Synthetic Pocket"
Yesterday, I was so busy and tired that I didn't have chance to write a post about a new paper I read. So, I missed a day in my #chempaperaday challenge. Anyway, so this is another paper about alkyne C-H activation with several cool figures.
DOI: 10.1021/ja412582k
Wednesday, February 12, 2014
Bond dissociation energy of KCl
So, I decided to do a practice and calculate the bond dissociation energy of KCl to compare it with the experimental data. As you can see I am very close to the actual value. Please feel free to check my calculation and correct me. Or if there is another way to calculate it, please teach me how to do that too.
#chempaperaday Day 27/365: "Iridium Porphyrins in CD3OD: Reduction of Ir(III), CD3−OD Bond Cleavage, Ir−D Acid Dissociation and Alkene Reactions"
I have never seen iridium porphyrins before (I haven't seen rhoidum ones too.). So, it is interesting for me to read this.
http://pubs.acs.org/doi/ipdf/10.1021/ic400240b
DOI: 10.1021/ic400240b
Due to my lack of knowledge, there are a few things that I couldn't understand. For example, the article says "Iridium(III) porphyrins are thus only metastable reagents for substrate reactions in strongly basic methanol." I have no idea what "basic methanol" is.
http://pubs.acs.org/doi/ipdf/10.1021/ic400240b
DOI: 10.1021/ic400240b
Due to my lack of knowledge, there are a few things that I couldn't understand. For example, the article says "Iridium(III) porphyrins are thus only metastable reagents for substrate reactions in strongly basic methanol." I have no idea what "basic methanol" is.
Tuesday, February 11, 2014
#chempaperaday Day 26/365: "Teaching the Jahn–Teller Theorem: A Simple Exercise That Illustrates How the Magnitude of Distortion Depends on the Number of Electrons and Their Occupation of the Degenerate Energy Level"
I started to read this 2 weeks ago. But, I had other stuff and forgot to finish it. Tonight it's done. I am wondering if any undergraduates do these exercises. Seems like overkill to me. Maybe good for computational skills.
http://pubs.acs.org/doi/abs/10.1021%2Fed300295r
DOI: 10.1021/ed300295r
Monday, February 10, 2014
#chempaperaday Day 25/365: "Designing Hydrolytic Zinc Metalloenzymes"
Zinc is one of the most common transition metal ions in biological systems. As you can expect, it is found as Zn(II) and acts as a strong Lewis Acid providing strong binding interactions. It can be found in several proteins and I can list at least two of them without looking at any textbook: carbonic anhydrase and alcohol dehydrogenase. I can add the zinc fingers too. But, they are not proteins although they can be found in several proteins.
Actually 6 months ago, I made an animation on PyMOL about carbonic anhydrase II and here it is:
I tried to link my youtube video, but it didn't work. Instead I uploaded the original file. Hope that works.
Today I am reading a great review on zinc metalloenzymes and to be honest I haven't finished reading yet. But, it will be over by midnight. I hope you enjoy it.
http://pubs.acs.org/doi/abs/10.1021%2Fbi4016617
DOI: 10.1021/bi4016617
Sunday, February 9, 2014
#chempaperaday Day 24/365: “Biologically Inspired Stealth Peptide-Capped Gold Nanoparticles"
I did a literature search and a brief review on gold nanoparticles (GNPs) as an assignment. So, I was lucky enough to read about them. When I first learned that some reactions take a few minutes to prepare the GNPs, I was so surprised. At first, I couldn't understand and asked to myself how is it possible to build a structure like that in a few minutes. Obviously, as I read more, I saw what's happening there. Making them is not the problem. Making them at the size you want, characterizing and making them function is the problem as far as I can understand.
I want to let some of the readers (who are not in chemistry) know that GNPs are not high-tech compounds of the 21st century. If you read some review papers, you will see that they go back to a few thousand years ago. People used them as therapeutics without knowing that the solutions they drank had GNPs. Now, the area that we can use them expanded. Because of their chemical inertness and photophysical properties they are excellent tools for imaging, delivery etc. Of course they have other issues.
So, I read this paper today and I skipped the experimental section. In this article, being inspired by the surfaces of most proteins (they tend to be charged, polar), the researchers capped the GNPs with a peptide sequence that consists of three parts. Obviously the part that binds to Gold is made of prolines and a cysteine (sulfur-hard/soft acid base theory). Then they tested the stability of the GNPs.
Saturday, February 8, 2014
#chempaperaday Day 23/365: “What are the Electrons Really Doing in Molecules?”
Today I read this article (free) about R.S.Mulliken that was written by a UMASS lecturer. Mulliken was an American chemist and a physicist.
He is very well known for his work and development of Molecular Orbital Theory. Anybody who has taken a course on Symmetry and Group Theory will also remember his name from Mulliken Symbols (A, B, E, T).
For his work on molecular orbital theory in particular he was awarded a Nobel Prize in Chemistry.
I know today's paper is not one of those journal papers, but hey that's what I read and it IS about chemistry. So, I guess it counts.
Electrons and Chemical Bonding by Harry B. Gray
I borrowed this book from the library yesterday and I thought it would help me to review my undergrad level knowledge of bonding. It did MORE!
The book is based on Harry B. Gray's lectures when he was in Columbia University. It's great overall. Although I just wanted to read it for a review, I liked it so much that I ordered one online so that I can own mine and highlight it. Instead of reading the book fast, I started to take notes like I am listening to a lecture. I've never seen such a nice and simple way of explaining bonding in textbooks.
02/08/1834 - Happy Birthday Mendeleev !
Of all the chemists I admire, Dmitri Mendeleev could be my favorite one. In a very well known and I guess the most common photo of him, we can see him with his long hair and beard. When I look at this photo, I can see the wise man in his eyes. He is sitting at his desk with a bunch of papers (chemistry journals maybe?) and probably talking to the interviewer. Behind his desk, there is a library full of books and I would like to learn what he read.
I will not go into the details of his life. One of the several books I've read is Mendeleyev's Dream and you can read my post about it here. I think you can read enough details about his life in the relevant chapter. I also have a bunch of books in my amazon shopping cart and they are waiting for their turn to be read.
Anyway, what really fascinating about Mendeleev is that he fully understood the periodic law and the properties of the elements that were known at that time. He was so good at this that he went further and predicted a few elements. He also stood up when there were some results that conflicted with his predictions. When Lecoq discovered Gallium (ekaaluminum) he reported different properties than Mendeleev had previously predicted. Instead of correcting his predictions with the experimental data, Mendeleev wrote to Lecoq and asked him to repeat the experiments. In the end, Mendeleev was right!
I think a few key elements that shaped his future in his career are the chemistry conference of 1860 Karlsruhe and his work/study with Kirchoff and Bunsen. He was also a man that did not really get along well with the government. He tried to stand by his people instead of the regime and had troubles because of his political views. But, he did not lose. At least he did what he could.
I also think that although some of his ideas seem unacceptable for some people, they are mostly correct. "We could live at the present day without a Plato, but a double number of Newtons is required to discover the secrets of nature, and to bring life into harmony with the laws of nature."
Today is his birthday and I am so happy that a man like him is recognized as the "father" of the periodic table. He was not awarded a Nobel Prize maybe, but at least he will be remembered forever with element 101: mendelevium.
More about him:
http://www.rsc.org/education/teachers/resources/periodictable/pre16/develop/mendeleev.htm
More about him:
http://www.rsc.org/education/teachers/resources/periodictable/pre16/develop/mendeleev.htm
Friday, February 7, 2014
#chempaperaday Day 22/365: "ReI–IrI bimetallic complexes based on a bis(chelating) ligand composed of 1,10-phenanthroline and N-heterocyclic carbene: Coordination chemistry and their application for optical indicator of CO gas"
I've read some papers about bimetallic complexes. But, I tried to find a new one for tonight. It is here:
I am not sure if it is free, but looks like I was able to view it without using my school's access. Maybe I was already logged in. Don't know.
So, they prepared a new ligand and three metal complexes (two of them bimetallic) with it. Maybe I'll do one of these one day.
Looks like they couldn't obtain crystals for the bimetallic complexes. They only report the ligand and the mono complex.
Thursday, February 6, 2014
#chempaperaday Day 21/365: "Structural Rearrangement Through Lanthanide Contraction in Dinuclear Complexes"
Another crazy day and I am so tired. Just got home and read this.
This is what I understood:
As we go across the periodic table atoms/ions have smaller radii (this one is known by everyone). For lanthanides, this decrease in radii cause change in geometry and coordination. So, they prepared series of complexes and investigated the change in their geometry and this fit well with the lanthanide contraction.
For coordination chemistry people, there are a few less common (at least for me) geometries such as "monocapped square antiprismatic" and "dodecahedron."
A beautiful structure.
Wednesday, February 5, 2014
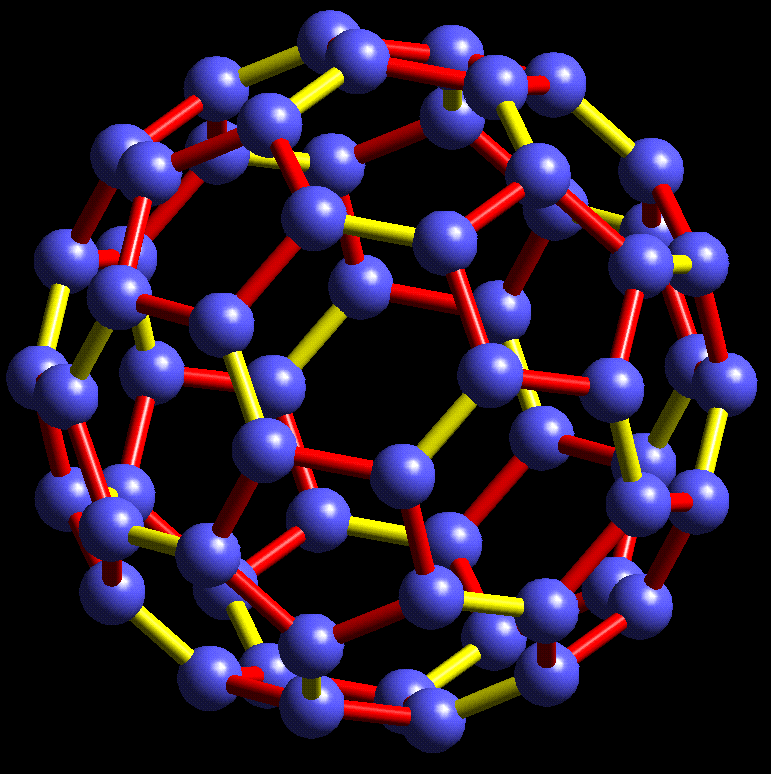
#chempaperaday Day 20/365: Buckministerfullerene
Today I wanted to read about this highly interesting molecule which is an allotrope of carbon. I learned that it was discovered accidentally discovered while the researchers doing experiments. They detected this stable cluster and found out what it was and suggested a structure.
Also the names they considered other than buckministerfullerene were ballene, spherene, soccerene and carbosoccer according to the paper. Honestly, I didn't like any of them except carbosoccer. Three of the authors were awarded the Nobel Prize in Chemistry in 1996.
I am surprised to see that it is a very short paper. Maybe there is a more detailed paper. I don't know.
Another challenge - Clayden's Organic Chemistry Textbook
This semester I have a lot of "free" time. I have only two courses. So, I spend most of my time by reading, studying and finishing my research (at least trying).
One of the MANY goals I set is to try to fully understand Clayden's organic chemistry textbook. Throughout my studies, I have used this book as a reference and source. But, all my attempts to really read it failed due to its unusual (for me) order of chapters and subjects. I know it is a great book, but I still don't like the way it was designed. But, having finished all the organic, inorganic and physical chemistry courses, I know that I can understand much more than I previously did. I am also sure that this will help me in graduate school (if I end up being in one).
One of the MANY goals I set is to try to fully understand Clayden's organic chemistry textbook. Throughout my studies, I have used this book as a reference and source. But, all my attempts to really read it failed due to its unusual (for me) order of chapters and subjects. I know it is a great book, but I still don't like the way it was designed. But, having finished all the organic, inorganic and physical chemistry courses, I know that I can understand much more than I previously did. I am also sure that this will help me in graduate school (if I end up being in one).
My favorite mug (for now)
Well, it looks like that I have had my first anniversary as an ACS member. It is my favorite mug now and makes me drink coffee more often.
Tuesday, February 4, 2014
#chempaperaday Day 19/365: "Hitler's Gift and the Era of Biosynthesis"
It was such a busy day for me. I was doing my reactions as planned, but then the possibility of having a snow day tomorrow made me realize that I have to quit/modify them just in case I won't be able to be in the lab tomorrow. Got home tired and had my dinner and read this amazing article (free).
I have a post about the book Hitler's Scientists and I learned a lot from that book. Hundreds of scientists from different fields had to/chose to leave Germany during the first half of 20th century. So, this article was published in the Journal of Biological Chemistry. It's written to "celebrate the centennary of JBC" and summarizes the interesting lives and works of three scientists.
The first scientist is Fritz Lipmann. What I liked about the part on his life is the author's way of pointing the importance of chemistry : "Lipmann realized that the most fruitful approach to biological problems was through chemistry." Another detail that I think is important in his career is that he was given "complete freedom to follow his ideas" at MGH.
Second important name is Rudolph Schoenheimer. Like Lipmann, he spent some years to learn chemistry and he is famous for tagging molecules with isotopes. I knew that many analytical techniques were not available at that time. But, learning that they used "combustion" and measuring density of compounds made me realize that those people were great scientists.
The third scientist is Konrad Bloch who is also a Nobel Prize winner. He became interested in science by an organic chemistry lecture. An interesting detail about his immigration is that he showed his acceptance letter to obtain a visa. But, in fact he did not have any funding. I believe he wouldn't have gotten the visa without a proof of financial support. He was in Schoenheimer's lab. But, after his death the group members chose their projects by a "lottery" though he is not really sure if this was the way they chose them. A fun fact about his life is that he went to Bermuda to collect a molecule that is abundant in shark livers. But, he realized that it's not an easy task and he came back.
I think this article is a fantastic read. It's a history of chemistry, medicine and biochemistry written in a beautiful style.
I have a post about the book Hitler's Scientists and I learned a lot from that book. Hundreds of scientists from different fields had to/chose to leave Germany during the first half of 20th century. So, this article was published in the Journal of Biological Chemistry. It's written to "celebrate the centennary of JBC" and summarizes the interesting lives and works of three scientists.
The first scientist is Fritz Lipmann. What I liked about the part on his life is the author's way of pointing the importance of chemistry : "Lipmann realized that the most fruitful approach to biological problems was through chemistry." Another detail that I think is important in his career is that he was given "complete freedom to follow his ideas" at MGH.
Second important name is Rudolph Schoenheimer. Like Lipmann, he spent some years to learn chemistry and he is famous for tagging molecules with isotopes. I knew that many analytical techniques were not available at that time. But, learning that they used "combustion" and measuring density of compounds made me realize that those people were great scientists.
The third scientist is Konrad Bloch who is also a Nobel Prize winner. He became interested in science by an organic chemistry lecture. An interesting detail about his immigration is that he showed his acceptance letter to obtain a visa. But, in fact he did not have any funding. I believe he wouldn't have gotten the visa without a proof of financial support. He was in Schoenheimer's lab. But, after his death the group members chose their projects by a "lottery" though he is not really sure if this was the way they chose them. A fun fact about his life is that he went to Bermuda to collect a molecule that is abundant in shark livers. But, he realized that it's not an easy task and he came back.
I think this article is a fantastic read. It's a history of chemistry, medicine and biochemistry written in a beautiful style.
Monday, February 3, 2014
#chempaperaday Day 18/365: "High-Nuclearity 3d–4f Clusters as Enhanced Magnetic Coolers and Molecular Magnets"
A few months ago, I became interested in molecular magnets. I tried to read some about these complexes. But, I didn't really understand much. I think one of the principles is to get an f block element with a d block and make a cluster. This interaction helps the complex to show desired magnetic properties. I might be wrong. But, I don't think I am so wrong. Anyway, I will probably learn more about them in the future. One of the reasons I like them is that they have beautiful structures like this one:
So this paper is about a cluster that could be used in "magnetic cooling."Did I understand everything? Of course not. But, as I read papers, I keep things in my mind that might help me one day. For example, the authors say that the bridging carbonate ions are thought to be formed by the absorption of "atmospheric CO2 by the reaction mixture."
Sunday, February 2, 2014
Subscribe to:
Comments (Atom)